Ap chem stoichiometry practice problems – Delve into the captivating realm of AP Chemistry Stoichiometry Practice Problems, where the intricacies of chemical reactions unravel. This comprehensive guide empowers you to conquer the complexities of stoichiometry, equipping you with the knowledge and skills to navigate chemical calculations with precision and confidence.
Throughout this exploration, you will embark on a journey that unravels the secrets of balancing chemical equations, performing stoichiometry calculations, and determining limiting reactants. You will delve into the significance of percent yield, explore the principles of titration, and unravel the mysteries of gas and solution stoichiometry.
1. Balancing Chemical Equations
Chemical equations are representations of chemical reactions that show the reactants and products involved. Balancing chemical equations is essential to ensure that the number of atoms of each element is the same on both sides of the equation. This is necessary because chemical reactions must follow the law of conservation of mass, which states that matter cannot be created or destroyed.
To balance chemical equations, the following steps can be followed:
- Write the unbalanced equation.
- Identify the atoms that are not balanced.
- Adjust the coefficients in front of the chemical formulas to balance the atoms.
- Check the equation to make sure that all atoms are balanced.
It is important to note that the coefficients in front of the chemical formulas represent the number of moles of each reactant and product. Therefore, balancing chemical equations also ensures that the stoichiometry of the reaction is correct.
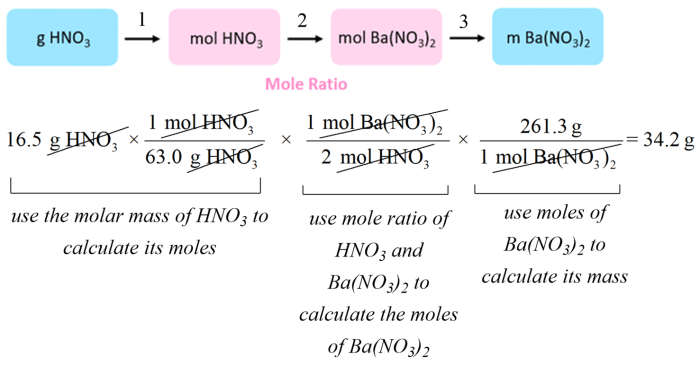
2. Stoichiometry Calculations

Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Stoichiometry calculations allow us to predict the amount of reactants and products that are involved in a reaction.
To perform stoichiometry calculations, the following steps can be followed:
- Write the balanced chemical equation for the reaction.
- Identify the mole ratio between the reactant and product of interest.
- Multiply the known amount of reactant or product by the mole ratio to find the unknown amount.
Stoichiometry calculations are essential for a variety of applications, such as predicting the yield of a reaction, determining the limiting reactant, and calculating the concentration of a solution.
3. Limiting Reactants
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, limiting the amount of product that can be formed. To determine the limiting reactant, the following steps can be followed:
- Calculate the number of moles of each reactant.
- Divide the number of moles of each reactant by its stoichiometric coefficient.
- The reactant with the smallest value is the limiting reactant.
The limiting reactant is important because it determines the maximum amount of product that can be formed. Once the limiting reactant is consumed, the reaction will stop, even if there is still excess of other reactants.
4. Percent Yield
The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100%. The theoretical yield is the amount of product that would be formed if the reaction went to completion, assuming 100% efficiency.
The percent yield can be calculated using the following formula:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
The percent yield is important because it tells us how efficient a reaction is. A high percent yield indicates that the reaction is efficient and that there is little waste. A low percent yield indicates that the reaction is inefficient and that there is a lot of waste.
FAQ Summary: Ap Chem Stoichiometry Practice Problems
What is the significance of stoichiometry in chemistry?
Stoichiometry provides a quantitative framework for understanding the relationships between reactants and products in chemical reactions, enabling precise predictions of reaction outcomes and the determination of optimal reaction conditions.
How can I improve my skills in solving stoichiometry problems?
Practice is key! Engage in solving diverse stoichiometry problems, paying meticulous attention to balancing chemical equations and applying mole ratios accurately. Utilize dimensional analysis to convert between different units and ensure the consistency of your calculations.
What are some common mistakes to avoid when performing stoichiometry calculations?
Ensure proper balancing of chemical equations, avoiding the introduction of errors that can propagate throughout the calculations. Pay attention to units and their conversion, as mismatched units can lead to incorrect results. Be cautious of rounding errors, especially when dealing with small quantities or large numbers.