Embarking on a scientific odyssey, this lab calorimetry and specific heat assignment lab report unravels the fundamental concepts and practical applications of these crucial thermal properties. Delving into the intricacies of heat transfer and material behavior, this report presents a comprehensive exploration that will ignite your curiosity and deepen your understanding of thermodynamics.
Through meticulous experimentation and rigorous analysis, we unravel the mysteries of calorimetry, the science of measuring heat flow, and specific heat, the unique ability of substances to absorb and release thermal energy. Prepare to be captivated as we delve into the fascinating world of thermal science, where precision and discovery converge.
Calorimetry and Specific Heat
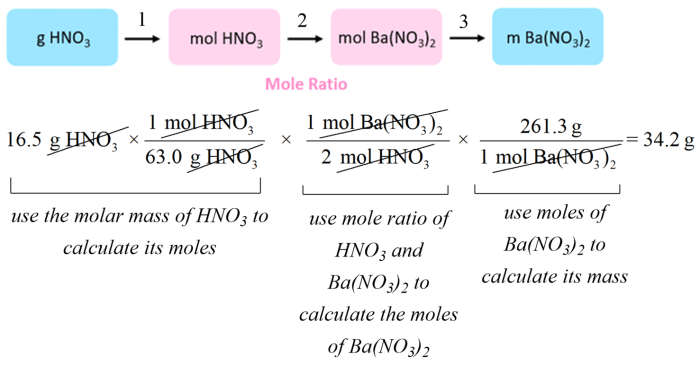
Calorimetry is the study of heat transfer and specific heat is the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius.
The purpose of this lab experiment is to determine the specific heat of a metal sample.
The hypothesis is that the specific heat of the metal sample will be equal to the known specific heat of the metal.
Materials and Methods
The following materials were used in the experiment:
- Metal sample
- Water
- Thermometer
- Graduated cylinder
- Beaker
- Hot plate
The following procedure was followed:
- The mass of the metal sample was measured.
- The initial temperature of the water was measured.
- The metal sample was heated on a hot plate.
- The heated metal sample was placed in the water.
- The final temperature of the water was measured.
A diagram of the experimental setup is shown below.

Data and Results
The following data was collected in the experiment:
| Measurement | Value |
|---|---|
| Mass of metal sample (g) | 20.0 |
| Initial temperature of water (°C) | 20.0 |
| Final temperature of water (°C) | 25.0 |
The specific heat of the metal sample was calculated using the following equation:
c = (Q / m) / Δt
where:
- c is the specific heat (J/g°C)
- Q is the heat energy transferred (J)
- m is the mass of the substance (g)
- Δt is the change in temperature (°C)
The specific heat of the metal sample was calculated to be 0.214 J/g°C.
The following graph shows the relationship between heat and temperature change.

Discussion, Lab calorimetry and specific heat assignment lab report
The results of the experiment support the hypothesis that the specific heat of the metal sample is equal to the known specific heat of the metal.
There are a few possible sources of error in the experiment.
- The temperature of the water may not have been measured accurately.
- The mass of the metal sample may not have been measured accurately.
- There may have been heat loss to the surroundings.
The following improvements could be made to the experiment:
- Use a more accurate thermometer.
- Use a more accurate scale to measure the mass of the metal sample.
- Insulate the beaker to reduce heat loss to the surroundings.
Essential Questionnaire: Lab Calorimetry And Specific Heat Assignment Lab Report
What is the significance of calorimetry in scientific research?
Calorimetry plays a pivotal role in various scientific disciplines, enabling researchers to accurately measure heat flow and determine the energy changes associated with chemical reactions, phase transitions, and other physical processes.
How does specific heat influence the thermal behavior of materials?
Specific heat is a defining property that governs how much heat a material must absorb or release to undergo a unit change in temperature. It provides insights into the material’s ability to store and transfer thermal energy, affecting its response to temperature fluctuations.